Objective:
To study the adsorption of iodine from
solution anduse Langmuir equation to
estimate the surface area of activated charcoal sample.
Theory:
Adsorption is a process where free
moving molecules of a gaseous or solutes of a solution come close and attach
themselves onto the surface of the solid. The attachment or adsorption bonds
can be strong or weak, depending on the nature of forces between adsorbent
(solid surface) and adsorbate (gas or dissolved solutes). When adsorption
involves only chemical bonds between adsorbent and adsorbate, it is recognized
as chemical adsorption or chemisorption. Chemical adsorption or chemisorptions
acquires activation energy, can be very strong and not readily reversible.
When the reaction between adsorbent
and adsorbate is due solely to van der Waals forces, this type of adsorption is
known as physical adsorption or van der Waals adsorption. This process is
non-specific and can occur at any condition. This type of adsorption is
reversible, either by increasing the temperature or reducing the pressure of
the gas or concentration of the solute.
Chemical adsorption generally
produces adsorption of a layer of adsorbate (monolayer adsorption). On the
other, physical adsorption can produce adsorption of more than one layer
adsorbate (multilayer adsorption). Nevertheless, it is possible that chemical
adsorption can be followed by physical adsorption on subsequent layers. For a
particular adsorbate/adsorbate, the degree of adsorption at a specified
temperature depends on the partial pressure of the gas or on concentration of
the adsorbate for adsorption from solution. The relationship between the degree
of adsorption and partial pressure or concentration is known as adsorption isotherm.
The studies of types of isotherm with temperature can provide useful
information on the characteristics of solid and the reactions involved when
adsorption occurs.
In adsorption from solution,
physical adsorption is far more common than chemisorption. However,
chemisorptions is sometimes possible, for example, fatty acids are chemisorbed
from benzene solutions on nickel and platinum catalysts.
Several factors will influence the
extent of adsorption from solution and is summarized in the table below.
Determination of Surface Area of
Activated Charcoal via Adsorption from Solution
Determination
of surface area of powder drug is important in the field of pharmacy and
colloidal science as surface area is one of the factors that govern the rate
dissolution and bioavailability of drugs that are absorbed through the
gastrointestinal tract.
Adsorption
measurement can be used to determine the surface area of solid. There are two
methods to measure the surface area which are Langmuir and B.E.T (Brunauer,
Emmett and Teller). In this experiment, adsorption of iodine from solution is
studied and Langmuir equation is used to estimate the surface area of activated
charcoal sample.
Materials and apparatus :
12 conical
flasks, 6 centrifuge tubes, measuring cylinders, analytical balance, Beckman
J6M/E centrifuge, burettes, retort stand and clamps, paster pipettes, iodine
solutions, 1%w/v starch solution, 0.1M sodium thiosulphate solution, distilled
water and activated charcoal.
Procedure:
Using burettes or measuring cylinders,
12 conical flasks (labeled 1-12)are
filled with 50ml mixtures of iodine solutions (A and B) as stated in the table
1.
Table 1: Solution A: Iodine (0.05M)
Solution B: Potassium Iodide (0.1M)
Set 1: Actual concentration of
iodine in solution A (X)
For flask 1 - 6:
1.
1- 2 drops of starch solution
are added as an indicator.
2.
The solution is then titrated
using 0.1 M sodium thiosulphate solution until the colour of the solution
change from dark blue to colourless.
3.
The volume of the thiosulphate
used was recorded.
Set
2: Concentration of iodine in solution A at equilibrium (C)
For flask 7-12:
1.
0.1g of activated charcoal is
added.
2.
The flask is capped tightly.
The flask is swirled every 10 minutes for 2 hours.
3.
After 2 hours, the solutions
are transferred into centrifuge tubes and they are labeled accordingly.
4.
The solutions are centrifuged
at 3000rpm for 5 minutes and the resulting supernatant is transferred into the
new conical flask. Each conical flask is labeled accordingly.
5.
Steps 1, 2 are 3 were repeated
as carried out for flask 1-6 in set 1.
Results:
Questions:
1.
Calculate N for iodine in each flask.
2. Plot amount of iodine adsorbed (N) versus balance concentration of
solution (C) at equilibrium to obtain adsorption isotherm.
3.
According to Langmuir theory, if there is no more than a monolayer
of iodine adsorbed on the charcoal,
Calculation:
From the graph
C/N versus C, 1/KNmobtained is 4.
Number of iodine
molecule adsorbed on the monomolecular layer
= Nm
× 0.1g charcoal × Avogadro no.
= 4.375×10-3
× 0.1 × 6.023 × 1023
= 2.635 × 1020molecules
Nm =
4.375×10-3 mole/gram ; 1 mole iodine = 2×126.9g
Weight of iodine
= Nm × 0.1g × (2×126.9g)
= 4.375×10-3 × 0.1 × (2×126.9)
= 0.111g
Thus, surface
area of charcoal can be calculated. Since surface area covered by one adsorbed
molecule is 3.2 × 10-19 m2,
4. Discuss the results of the experiment. How do you determine
experimentally that equilibrium has been reached after shaking for 2 hours?
Equilibrium has been reached when the solution becomes homogenous
and has no more colour changed.
Discussions:
Adsorption
is usually described using isotherms which are the amount of adsorbate on the
adsorbent as a function of its pressure for gases or concentration for liquid
at constant temperature. The quantity adsorbed is always normalized by the mass
of the adsorbent in order to allow comparison of different substances.
Langmuir
isotherm adsorption is based on the theoretical equation on adsorption based on
the short distance forces that present between molecules. It is a
semi-empirical isotherm derived from a proposed kinetic mechanism. The equation
got four assumptions as below :
(a) The surface of the adsorbent is
uniform which all adsorption sites are equivalent. The gas molecules are
adsorbed at fixed site on the surface.
(b) The adsorbed molecules do not
react with each other.
(c) The thickness layer of gas
molecules being adsorbed is one molecule thick, the adsorbate molecule do not
deposit on other already adsorbed surface, only on the free surface of the
adsorbent.
(d) All adsorption occurs through
the same mechanism.
In
this experiment, iodine is the adsorbate while charcoal is the adsorbent. In
the set 1 experiment, titration method was used to calculate the concentration
of iodine. This is because the iodide ion and iodine molecule are in
equilibrium in the conical flask. Starch is used as an indicator in the
titration. The solution turn dark blue colour when starch is added as iodine
molecules are present. Then, when sodium thiosulfate is added, the iodine
molecule react with sodium thiosulfate to form sodium iodide.
I2 + 2Na2S2O3
→ Na2S4O6 + 2 NaI
When there is totally
noiodine molecule in the solution, the dark colour change to colourless. From
the equation, the moles of iodine can be calculated.
In the set 2 experiment, 0.1 g of activated charcoal was added
into flask 7-12 and capped tightly. The activated charcoal added is act as
adsorbent to adsorp the iodine molecule. Adsorption of iodine molecule on the
activated charcoal is a result from Van der Waal’s forces which exists between
molecules. The forces are extremely short ranged and therefore sensitive to the
distance between the carbon surface and the adsorbate molecule. They are also
addictive which the adsorption force is the total of all interactions between
all the atoms.
A large specific surface area is preferable for providing large
adsorption capacity, however, large internal surface area in a limited volume
will give rise to large numbers of small pores in between the adsorption
surface. The size of the pores will determine the accessibility of the
adsorbate molecules into the internal adsorption surface area of adsorbent.
Hence, the distribution of the micro pores size is an important property for
characterized the adsorptivity of adsorbents.
C/N = C/Nm +
1/KNm.
In this experiment, there are some errors and precautions that
need to be notified. There may be more than one layer of iodine molecules
adsorbed on the surface of the activated charcoal. Besides, the concentration
of solution may affected when swirling if the conical flask is not capped
tightly. So the conical flask should be capped tightly. Moreover, the volume
measured should also be accurate and avoid from parallax error as it may affect
the results. The conical flasks also have to be swirled constantly to allow all
charcoal molecules exposed to the mixture solution in order for adsorption to
take place.
Conclusion :
The surface area of the
activated charcoal is 759.64m2g-1.
References
:
1. 1. A.T.Florence and D.Attwood.Physicochemical Principles of Pharmacy, 3rd
edition,
1998. Macmillan Press LTD.
2.
http://en.wikipedia.org/wiki/Adsorption
3.
infohost.nmt.edu/~jaltig/Langmuir.pdf













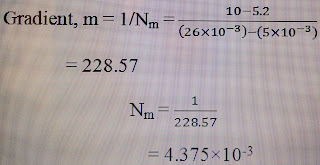


Nice post. Check out Mi Courier Services!
ReplyDelete